Research in the Sherman Group focuses on the pursuit of chemical strategies for capturing and storing solar energy. The challenge of developing stable and highly active photoelectrochemical systems that can use light energy to drive specific chemical reactions at an interface involves research work spanning several fields of chemistry including inorganic, organic, analytical, and materials chemistry. For students in the group, the multidisciplinary nature of the research provides exposure across diverse topics in chemistry and enables ample opportunity for collaboration both within the Department of Chemistry and Biochemistry at TCU as well as with research groups at other institutions. Synopses of the main research projects in the group are detailed below.
Photoelectrochemical Water Splitting
Much of the research work in the group focuses on the development of tandem junction dye-sensitized photoelectrochemical cells (DSPECs) capable of converting water to H2 and O2 with the only energy input from sunlight. The fabrication of photoelectrodes for use in a DSPEC combines light absorbing materials, wide band-gap semiconducting oxides such as TiO2 and SnO2, and molecular or nanoparticulate catalysts to drive water oxidation and hydrogen formation half reactions. Research directions include the development of novel water oxidation catalysts and strategies to improve the stability and solar energy conversion efficiency of this approach to harnessing solar energy.
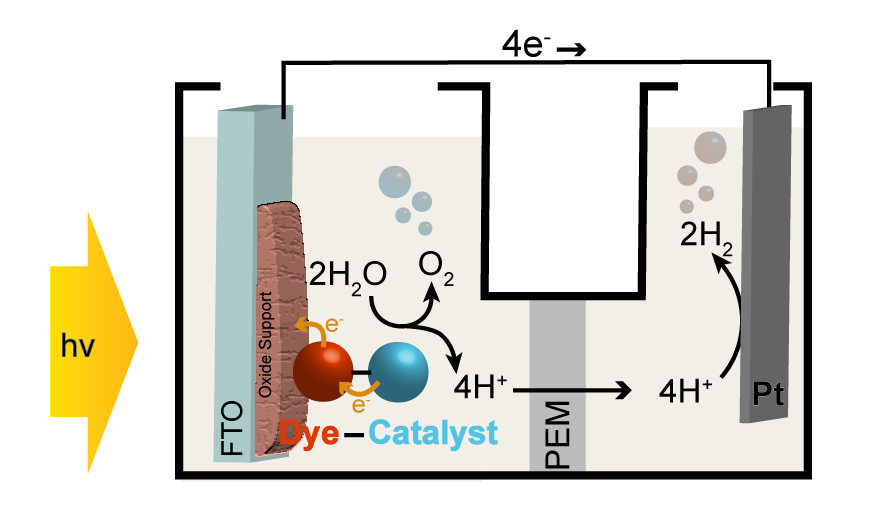
General depiction of a dye-sensitized photoelectrochemical cell.
Electrochemically Prepared Light-Active Electrode Coatings
The development of photoelectrodes with robust and highly active surface films is critical to the success of DSPECs as a viable solar energy platform. While there are several strategies for preparing photoanode or photocathode surfaces, one particular method of interest is the use of electropolymerization to form interfacial structures composed of chromophore, catalyst, and linking moieties. We are investigating the preparation of chromophore–catalyst films using dye species and colloidal iridium oxide (IrOx•nH2O) nanoparticles which are specifically functionalized to enable electrochemically driven deposition at the electrode surface. This approach would enable control over the extent of deposition of each component and the introduction of spacer layers or other chemical functional groups which could have a profound influence on the reactivity and stability of the electrode interface.


